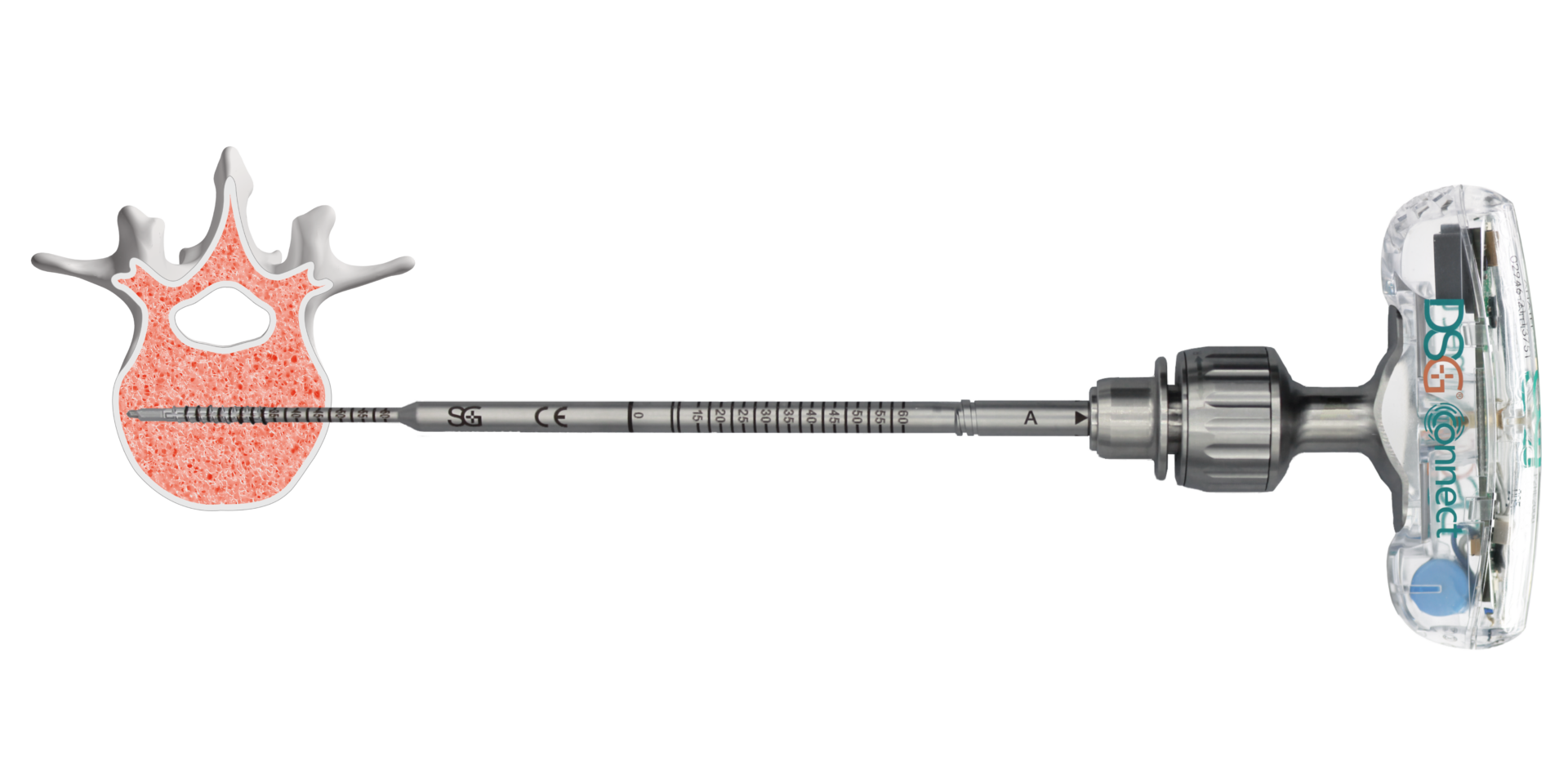
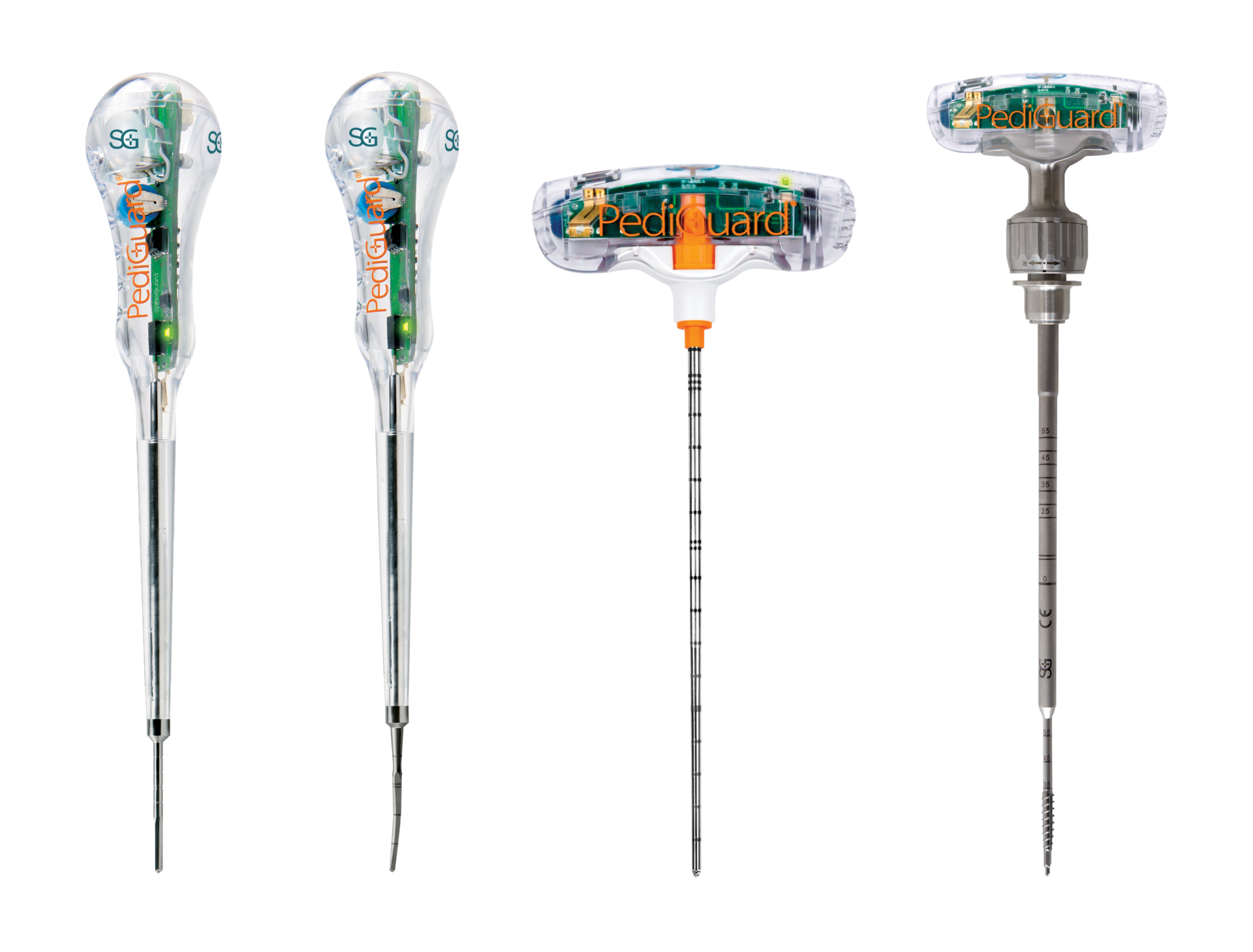
Secure Implant Placement
The Benefits of DSG Technology
Pedicle Screw
Placement Accuracy
100% Medial Pedicle
Breach Anticipation1
Real-Time
Feedback
Less radiation
exposure

Larry Khoo, MD
Co-founder of SMISS & ISASS
The main benefits of PediGuard are those that have always been there: active detection at the tip of the probe…which allows us to know that whatever trajectory the fluoroscopy or navigator is telling us is going to be safe and not damage any of the local neural structures.
References
1 – Williams J. Anticipation of vertebral pedicle breach through dynamic surgical guidance. Coluna/Columna. 2014;13(3):210-3
Vous avez des questions ?